Cmdh Slot Request Form
- Cmdh Slot Request Form 9465
- Cmdh Slot Request Form Sss
- Cmdh Slot Request Forms
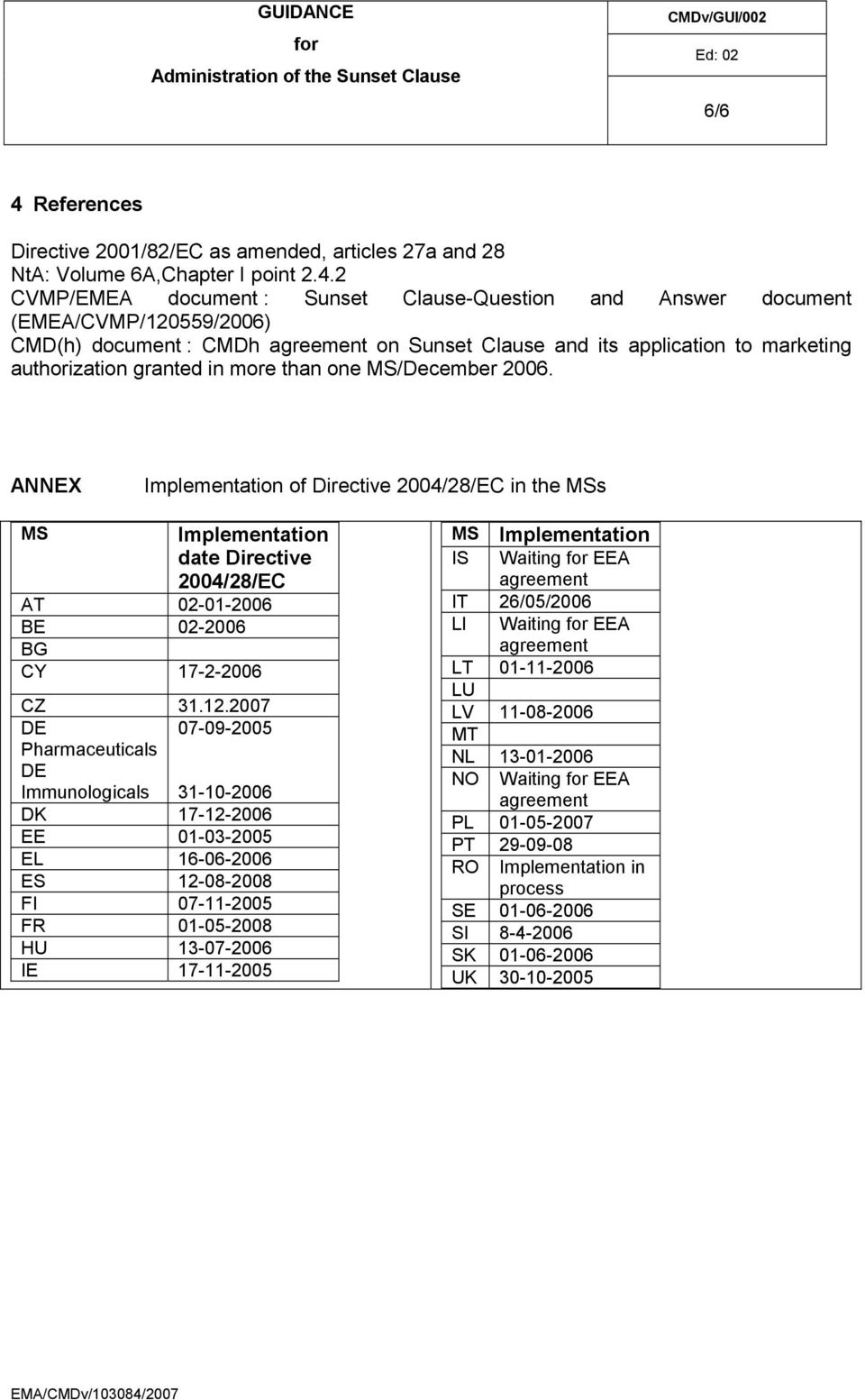
- Cmdh Slot Request Form Template
Form to Request Schedule Slots To Change any of the information on an existing schedule slot, please fill out the following chart. You only need to fill out fields that will be changing. Opportunity title: Date of Schedule Slot to be changed (m/d/yy): New Date: New Start and End time: New Max # of Vols. The request for registration should include the registrant’s name and each trade name under which the registrant does business, the address of each of the registrant’s places of business, the street address in any state or possession of the United States where the gambling device records can be viewed, the officers’ or owners’ names.
Removal of Booking Change Request
As announced on 20 October 2020 Link to announcement
With effect from 23 October (Fri), we will no longer be accepting request of changes to your bookings. The intention of facilitating a Booking Change Request for the past months is to allow climbers some time to adapt to the online booking system and lifestyle changes post Circuit Breaker. All requests for changes submitted before 22 October (Thu), 23:59 will be processed and followed up with accordingly.
Moving forward, if you have made an incorrect booking or have a change in plans, please cancel your booking before making a new booking online. Here are the various ways you can choose to make the cancellation:
On the website:
You can submit a Cancellation Request Form online (12 hours prior to your booking time slot). It will take at least 1 working day for the request to be processed and you will receive a booking cancellation email.
For the ones that have created online profiles and logged in, you can now use the “Cancel booking” button (12 hours prior to your booking time slot) under “My Recent Online Bookings” and a cancellation email will be sent to you once it has been processed.
On the RGPro Connect App:
If you have downloaded the RGPro Connect App and made a booking via the app, you can now use the “Cancel booking” button (12 hours prior to your booking time slot) under “My Recent Online Bookings” to cancel your booking. A cancellation email will be sent to you once it has been processed. Do note that booking records on the app may reflect bookings you have made via the app only. If the booking you would like to cancel is not reflected on the app, you can submit a Cancellation Request Form here.
A reminder that each climber can book one confirmed climbing slot per day. Late cancellations/ double bookings/ no-shows will be subjected to penalties.
Decentralised Procedure (DCP) / Mutual Recognition Procedure (MRP)
At present there are two different procedures to apply for a marketing authorisation for the same medicinal product in the EU/EEA in more than one Member State:

- the Decentralised Procedure (DCP)
- the Mutual Recognition Procedure (MRP).
Cmdh Slot Request Form 9465
Both, MRP and DCP, are open for all applications for marketing authorisation which do not fall within thet mandatory scope of the Centralised Procedures. The MRP has to be chosen in the case a marketing authorisation for the same medicinal product has already been granted by a Member State of the EU/EEA, whereas the DCP is applicable if no marketing authorisation exists
In general, one Member State will be selected by the Applicant to carry out the procedure (Reference Member State, RMS) for both types of procedure. The application is based on the identical dossier submitted to all CMS. Apart from leading the administrative procedure, the RMS is responsible for preparing an Assessment Report (AR) which summarises the dossier presented by the applicant. The AR characterises and critically evaluates the medicinal product concerned with regard to its quality, safety and efficacy . This AR will be made available to all Concerned Member States (CMS) by the RMS and forms the basis for the evaluation by CMS. The Applicant also receives a copy of the AR, however without confidential information MS concerned by either the MRP or the DCP will have 90 days to accept the AR (MRP) or the draft AR (DCP) of the RMS and to issue a marketing authorisation. Further documents may have to be submitted by the applicant to clarify open questions and outstanding issues.
In the case a Member State concerned by the procedure is unable to accept the AR or draft AR on the basis of a “potential serious risk to public health” as defined in Article 29(1) of Directive 2001/83/EC as amended, and further elaborated in the Commission Communication (Official Journal C 133, 8/6/2006 p. 5 - 7) and the Annex to this Commission Communication (“Notice to Applicants, Volume 2 C - Regulatory ” ), the application will be forwarded for further discussion to the Coordination Group for Mutual Recognition and Decentralised Procedures (CMD(h)). If the CMD(h) is unable to resolve the issue within 60 days, the application will be sent to the CHMP for arbitration.
Notes on applying for a Decentralised Procedures (DCP):
A DCP must be requested in wirting. Please note the Guide to the Submission of Applications for Marketing Authorisation of Medicinal Products as well as the Guide to Submission of Applications for Marketing Authorisations of the Decentralised Procedure (DCP) (according to Art. 28(3) of Directive 2001/83/EC).
The Coordination Group for Mutual Recognition and Decentralised Procedures (CMDh) develops and publishes corresponding guidelines on the DCP on its website, where further information on the procedure can be found, e.g.
Note on submission of draft responses
If deficiencies are identified during the first assessment phase, the applicant shall be given the opportunity to amend his dossier (clock stop). In the case of many and/or serious deficiencies, the submission of draft responses for preliminary assessment by the RMS is recommended. Draft response documents submitted for pre-assessment when Germany is acting as RMS cannot be accepted via CESP. These should be submitted directly to the responsible case manager via e-mail or EudraLink.
See also Using the COMMON EU SUBMISSION PLATFORM (CESP) for electronic submissions to the BfArM
Notes on applying for a Mutual Recognition Procedures (MRP) and ‘Repeat Use’ Procedures (RUP):
Cmdh Slot Request Form Sss
A Marketing Authorisation Holder (MAH) can use the Mutual Recognition Procedure (MRP) for the same authorisation more than once after completion of a first MRP or a Decentralised Procedure (DCP) for the recognition of a marketing authorisation by other Member States (MS). This procedure is known as “Repeat Use” (RUP).

The MAH should send a written request for MRP or RUP to the BfArM including the completed forms as published on the CMDh website:
- Request for MRP/RUP for Medicinal Products for Human Use
- Appendix 1 to Request for MRP/RUP for Medicinal Products for Human Use
- Update Assessment report for Repeat use procedures
Cmdh Slot Request Forms
In this context, please also note the general information of the BfArM on the Submission of Applications for Marketing Authorisation of Medicinal Products.
Cmdh Slot Request Form Template
The Coordination Group for Mutual Recognition and Decentralised Procedures (CMDh) develops and publishes corresponding guidelines on the MRP and RUP on its website, where further information on the procedures can be found, e.g.